Antimicrobial Sensitivity:
Biofilm vs. Planktonic Cells
(Using the MBEC Assay System HTP1)
Instructor Version (go to Student Version)
| Subject Area(s) | Microbiology |
Intended Audience |
High school biology, independent study/science fair, introductory undergraduate microbiology, advanced college level microbiology |
Type |
Laboratory exercise |
Revision Date |
June 16, 2009 |
CONTENT
One of the most important properties of biofilms is the increased resistance to antimicrobial substances, both disinfectants and antibiotics. This resistance has implications for both clinical and industrial microbiology. This exercise illustrates a method for determining the resistance of biofilms to disinfectants and antibiotics and for comparing that resistance to planktonic cells of the same species. The exercise employs the MBEC – Assay System2,3,4,5,6 (Minimal Biofilm Eradication Concentration), also called the Calgary Biofilm Device and devised at the University of Calgary. The MBEC-Assay system HTP version is available through “innovotech”, suite 101, 2011-94st. Edmonton, Alberta, T6N1N1 Canada.
PREREQUISITES
Students should be able to define a biofilm, describe the differences between biofilm (surface-attached) and planktonic (free-floating) bacteria, and be able to describe why bacteria usually grow on surfaces. Students should be familiar with standard methods handling and transferring microbial cells with aseptic technique. Students should also be familiar with the concept of minimal inhibitory concentration (MIC) as applied to planktonic cells.
INSTRUCTIONAL OBJECTIVE
Given readily available materials and detailed instructions the student will be able to produce multiple biofilms on plastic surfaces. These biofilms and the planktonic cells which regularly slough off from them will be used to demonstrate the difference in susceptibility (if any) of a given microorganism in its biofilm and planktonic states after treatment with a disinfectant or an antibiotic. These susceptibility values are known as the minimal biofilm eradication concentration (MBEC) and the minimal inhibitory concentration (MIC), respectively.
INSTRUCTIONAL PROCEDURES
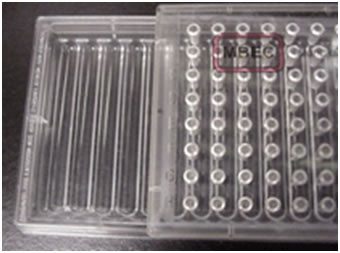
This exercise employs a new tool called the MBEC Assay System 2,3,4,5,6. This is a sterile plastic tray similar in design to the standard 96 well microtiter plate (see Figure 1). The lid of this plate has 96 plastic pins arranged in 8 rows of 12. Unlike the microtiter plate the base of the device does not have 96 individual wells, but instead has 12 channels into which the pins extend. With this design 96 pins can be exposed to a given culture simultaneously. During biofilm growth, the MBEC device is typically placed on a tilt or rocker table (10 rpm) so that the culture flows back and forth producing sheer forces that stimulate biofilm formation.
Teachers Note: If only a rotary shaker is available, MBEC Bioproducts manufactures another sort of plate that has the same lid and peg arrangement but has a 96 well base rather than the open tray design (PG Type plate). This plate design works as well as the open tray design for producing biofilms, but has the disadvantage that each well must be inoculated independently. On the other hand, this allows the use of more than one organism per plate.
Protocol:
a. Preparation of the culture
A suspension of the experimental organism is prepared by mixing a colony (or several identical colonies) from an overnight plate culture into an appropriate nutrient medium. For example, 1/10 strength LB broth 7 works well for Pseudomonas putida. Biofilm formation is stimulated by low nutrient conditions so a 1:10 dilution of media like LB or TSB2 (tryptic soy broth) is recommended.
The suspension should be visibly turbid, ideally equivalent to a McFarland standard of 1.0. This equates to approximately 3 X 108 bacteria per ml.
See reference 8 for instructions to prepare the appropriate McFarland standard.
Dilute this suspension 1:30 into 1/10 strength nutrient medium (e.g. 1/10 LB or TSB) to produce 31 ml of the diluted culture (ideally this should contain approximately 1 X 107 cells/ml).
b. Biofilm formation
Open a sterile MBEC™ plate. Add 22 ml of the suspension prepared in step A. The lid of the MBEC device is then secured to the base of the plate so that the 96 pegs are immersed in the culture medium. Place the MBEC device on a tilt or rocker table at 10 RPM so that the culture flows side to side along the short axis of the plate. The shear forces exerted on the pins results in the formation of more robust biofilms than a stationary culture. For a given organism, the cultures are incubated at the optimum temperature for 24 to 48 hours. After incubation, the lid contains 96 pins, each with an equivalent biofilm attached 2,3,4,5,6.
Teachers Note: If you are using the PG type plate with the 96 well base, each well should be inoculated with 200 μl of the bacterial suspension.
c. Preparation of the antimicrobial (see note above) agents
Preparation of the stock solutions
Prepare 5 ml of a 5.12 mg/ml solution of the desired antibiotic in sterile distilled water. Dispense this solution in 250 μl aliquots into sterile screw capped tubes or sterile micro-centrifuge tubes. Freeze these stock solutions until the day of the laboratory or prepare them fresh the day of the lab.
Preparation of the use dilutions
On the day of the laboratory dilute the 250 μl of stock solution 1:5 by adding 1 ml of sterile 1/10 strength Muller Hinton Broth2 (MHB) or Cation Adjusted Muller Hinton Broth2 (CAMHB) broth to the screw capped tube or micro-centrifuge tube. These tubes are the starting solution and one tube is given to each student or pair of students (1250 μl volume at 1024 μg/ml).
The dilution series
Each student or team of students should be assigned a series of 12 wells in an MBEC plate (Rows 1-12).
Into well 1, place 200 μl of 1/10 MHB. This is the zero control well.
Into well 2, place 200 μl of the undiluted antibiotic solution in 1/10 strength MH broth (1024 μg/ml of antibiotic).
Into wells 3 through 12, place 100 μl of MH broth.
Starting with well 3, add 100μl of the undiluted antibiotic solution. Pipette the solution up and down several times and remove 100 μl and place into well 4.
Repeat this process for wells 5 through 12. After well 12 is mixed, 100 μl of the solution in well 12 is removed and discarded.
Teachers Note: Buckets containing bleach (1:10 dilution with water) should be provided to receive the discarded material and used microtiter plates.
Finally, 100 μl of sterile 1/10 strength MH broth is added to each well (wells 3 to 12) bringing the final volume in each well to 200 μl.
The final concentrations in each well are:
| Well | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg/ml | 0 | 1024 | 512 | 256 | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 |
d. Challenging planktonic and biofilm cells with the antimicrobial see note agent
1. The MBEC lid is rinsed by immersing the pins into a 96 well plate in which each well contains 250 ml of sterile 0.9% saline (plate 1) (see Figure 2).
2. The lid of the MBEC assay device with its 96 biofilm coated pins is then lowered into the 96 well microtiter plate prepared above in step c. This plate is called the Challenge Plate (plate 2). Each row of twelve wells contains a dilution series of the selected antibiotic. The volume in each well should be adjusted to accommodate the volume of the well (typically 200 μl/well). In any event, the antimicrobic see note solutions should completely cover the biofilm on the pins.
The plates are incubated over night at the optimum temperature for the organisms selected (25 °C for P. putida) without shaking or the use of a tilt table.
During this overnight incubation, some cells have sloughed off the biofilm. Most of these cells rapidly revert to their planktonic phenotype. These cells are exposed to the antimicrobial agent in the planktonic state. Growth in any well (shown as turbidity), indicates that the planktonic cells have survived the antimicrobial treatment. This observation gives a minimal inhibitory concentration (MIC) or the minimal concentration that completely inhibited cell growth as indicated by a complete lack of turbidity. Cover these plates with a standard 96 well plate lid and refrigerate them plates until the next class period.
3. After the 24 hour incubation period, the MBEC lid is rinsed byplacing it in a 96 well microtiter plate in which each well contains sterile 0.9% saline (250 μl/well) (plate 3). This rinse removes the excess antimicrobial note agent. Rinse for one minute and then discard this wash plate in the buckets containing 1/10 Chlorox ™ solution
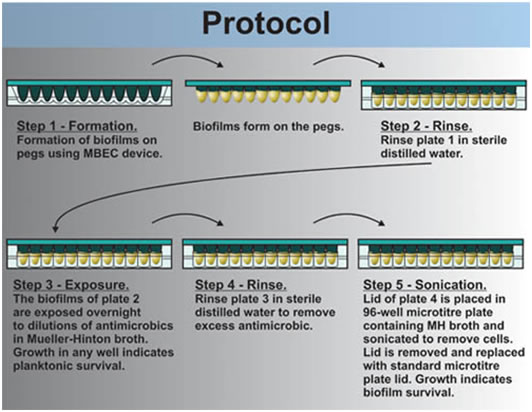
4. The washed MBEC lid is now placed in a 96 well plate in which each well contains 200 μl sterile 1/10 strength MHB or CAMHB with no antimicrobial note agent (plate 4). The biofilms are removed from the pegs by sonication. To do this, a pan containing about 3 mm of water is floated on the liquid surface of a sonic water bath. The MBEC lid and 96 well tray are placed in the pan and the device is sonicated on high for 5 minutes. l.The MBEC lid is now replaced by a standard microtiter plate lid and the MBEC lid containing the pegs is discarded into tye buckets of 1/10 Chlorox ™ solution.
The plates Mueller Hinton plates (Plates # 4) are incubated overnight at the optimum temperature for the organism selected. Growth in any well following incubation indicates that at least some of the cells in the biofilm attached to the pins have survived the antimicrobial note agent.
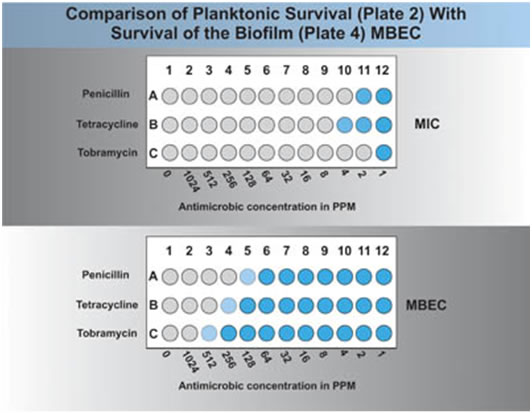
A visual comparison of plate 2 and plate 4 should indicate the relative resistance of planktonic and biofilm associated cells. If a plate reader is available this comparison can be made automatically (see Figure 3). A comparison of the MIC (plate 2) and the MBEC (plate 4) will give an idea of the difference in antimicrobial resistance between the planktonic cells and the biofilm associated cells. For example, in Fig 3 one can see that the organisms MIC was 2 ppm of the antibiotic or 2 μg/ml, while the MBEC was 128 μg/ml, a 64 fold increase in resistance of the biofilm cells over the planktonic ones.
MATERIALS AND EQUIPMENT PER TEAM OF STUDENTS
| 1 | Sterile MBEC™ lid and trough. Teachers note: several students or pairs of students can use the same MBEC device, thus reducing the cost of this exercise. |
| 4 | Standard 96 well microtiter plates |
| Mueller Hinton Broth (1/10 normal strength) for preparing stock solutions and antimicrobial note dilutions (sterile) | |
| Tryptic soy broth (sterile) 1/10 normal strength | |
| Samples of disinfectants and antibiotics (sterile) for testing. The number of antibiotic agents that might be used in this exercise is very large and might include penicillin G, amikacin, ampicillin, ciprofloxicin, vancomycin, tetracycline, streptomycin, and tobramycin. Antibiotics with limited water solubility or which are colored (rifampin) should probably be avoided. | |
| Sterile 0.9% saline | |
| Sterile pipettes, 1 ml size | |
| Automatic pipettes adjusted to 200 μl and 100 μl | |
| Sterile pipette tips appropriate for the above | |
| An overnight culture of Pseudomonas putida or other organism on solid medium such as tryptic soy agar. | |
| Gloves (latex, nitrile or equivalent) | |
| Platform rocker (tilt angle approx 9°) set at 10 excursions per minute | |
| Vortex mixer | |
| Sonic waterbath cleaner and small pan | |
| Sonic waterbath cleaner and small pan |
ASSESSMENT / EVALUATION
The student and the instructor can evaluate this exercise by a visual comparison of growth in the planktonic plate (plate 2) and the biofilm plate (plate 4). This comparison may also be carried out on a plate reader if one is available. A typical result would reveal that the planktonic cells are killed at a lower concentration of the antimicrobial note agent than the biofilm cells of the same organism.
FOLLOW-UP ACTIVITIES
This procedure may be linked to several other exercises including the “Bring ‘Em Back Alive” exercise. Students may compare the sensitivity of planktonic and biofilm associated cells in a biofilm they have isolated from some natural source. The MBEC device and the techniques described here can be used to produce biofilm cells for other exercises requiring either attached or dispersed cell suspensions.
REFERENCES
1. The MBEC Assay System is available through MBEC Biofilms Technology Ltd., Calgary Alberta, Canada.
2. H. Ceri, M.E. Olson, C Stremick, R.R. Read, D. Morck and A. Buret. 1999, The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms, Journal of Clinical Microbiology, Vol. 37, pp 1771-1776.
3. Bardouniotis, Elias, Howard Ceri and Merle E. Olson. 2003, Biofilm Formation and Biocide Susceptibility Testing of Mycobacterium fortuitum and Mycobacterium marinum, Current Microbiology, Vol. 46, pp 28-32.
4. H. Ceri, D.W. Morck, and M.E. Olson, 2001, Chapter 75. Biocide Susceptibility Testing of Biofilms, In Disinfection,, Sterilization and Preservation. 5th edition. S. Seymour editor. Lippincott, Williams & Wilkins, Baltimore, MD. pp. 1429-1437.
5. H. Ceri, M.E. Olson, D.W. Morck and D.G. Storey, 2006. Minimal Biofilm Eradication Concentration (MBEC) Assay: Susceptability Testing for Biofilms, In: Biofilms, Infection , and Antimicrobial Therapy, J.L. Pace, M.E. Rupp and R.G. Finch, Eds. Taylor & Francis, Boca Raton.
6. Merle E. Olson, Howard Ceri, Douglas W. Morck, Andre G. Buret, Ronald R. Read, 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics, Canadian Journal of Veterinary Research. Vol. 66 (2), pp 86–92.
7. MBEC™ Assay for High-throughput Screening (HTP) Using a 96-Peg Lid and Trough. This is the protocol distributed to clients who purchase the MBEC Device. Kindly supplied by M.E. Olson, Calgary Alberta, CA.
8. BD BBL™ Trypticase Soy Broth – Ca\t. No. 211768, BD Difco™ LB Broth, Lennox – Cat. No. 240230, BD BBL™ Mueller Hinton Broth – Cat. No. 211443, BD BBL™ Mueller Hinton Broth (Cation Adjusted) – Vat. No. 212322.
9. A complete description of the preparation of McFarland standards can be found at ⇑⇑⇑ http://www.microbiol.org/white.papers/WP.OD.htm.
Preparation of McFarland Standards. McFarland standards are a method of visually estimating bacterial concentrations by comparison of the bacterial suspension with the standard using the same light path (The bacterial titers were calculated for Gram negative organisms such as E. coli). Standards are made by mixing barium chloride solutions and sulfuric acid to produce a suspension of barium sulfate. A McFarland standard of 1 is approximately equivalent to a bacterial titer of 3 X 108. This can be prepared by mixing 0.1 ml of 1% anhydrous barium chloride with 9.9 ml of 1% H2SO4. This is approximately equivalent to an absorbance of 0.08-.1 in a spectrophotometer cuvette with a 1 cm light path and at 625 nm. McFarland standards should be kept in the dark and discarded after no more than six months. They should be shaken vigorously prior to use.
------------------------------------------------------------------------
Educational Program Curricula and Teaching Resources
Developed in collaboration with Dr. John Lennox, Penn State University-Altoona
NOTE: Links marked by the 3-arrow sign take you to non-Center for Biofilm Engineering web sites. The Center is not responsible for content of sites marked with the 3-arrow sign ⇑⇑⇑.
