What is the evidence that biofilm forms in chronic wounds?


Evidence from a CBE study
In collaboration with the Southwest Regional Wound Care Center, in Lubbock, Texas, the CBE examined acute and chronic wound samples from human volunteers for the presence of biofilms. The primary questions addressed by this research included:
- Are bacterial biofilms present in most, or all, persistent wounds?
- Are bacterial biofilms present in acute wounds?
Chronic wound samples were obtained from patients undergoing sharp debridement of persistent wounds as part standard wound care management. Samples were also collected from patients with acute wounds using standard surgical procedures to collect elliptical tissue sections with a minimum diameter of approximately 1 centimeter. The orientation of the tissue (i.e. top and bottom) was indicated using tissue dye. The samples were preserved in a phosphate-buffered formalin solution, transferred to a 70% ethanol solution, and shipped overnight to CBE. A total of 50 samples from persistent wound infections and 16 samples from acute wounds were obtained. Analysis of the samples included scanning electron microscopy (SEM) and light microscopic evaluation of Gram-stained tissue thin sections. Criteria for biofilm classification the included number and density of cells, large cellular aggregates were classified as biofilm while individual or small clusters of cells were not, and the presence of extracellular polymer substance (EPS) around the bacterial cells.
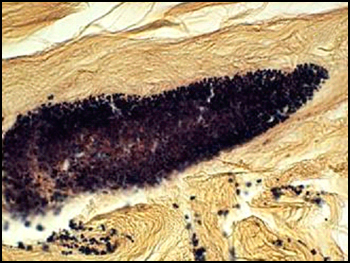
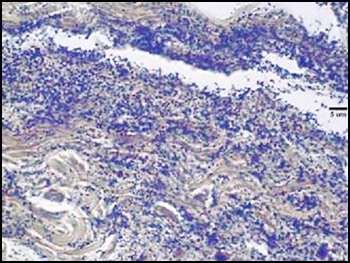
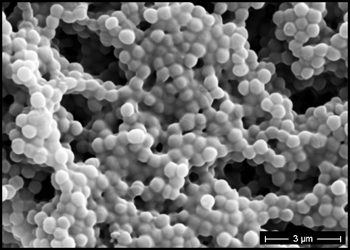
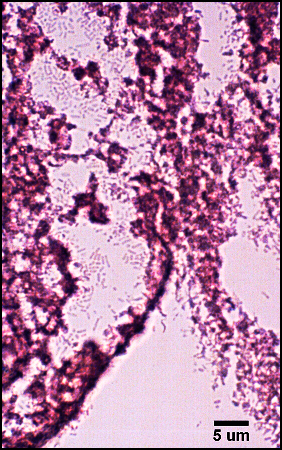
The predominant type of biofilm bacteria observed using light microscopy were Gram positive cocci (Figure 5). These results were in agreement with culture data which showed a predominance of Staphylococcus and Enterococcus from these patients. SEM examination of the samples confirmed the predominance of coccoid cells, which often appeared to be coated with EPS (Figure 6). Similar coatings were observed in pure culture biofilms of Staphylococcus aureus grown using the colony biofilm model (Figure 7).
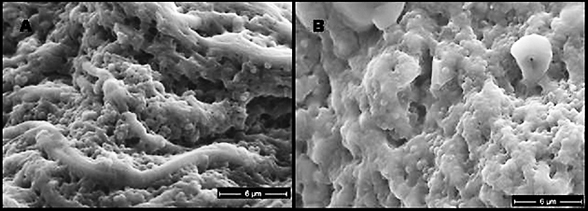
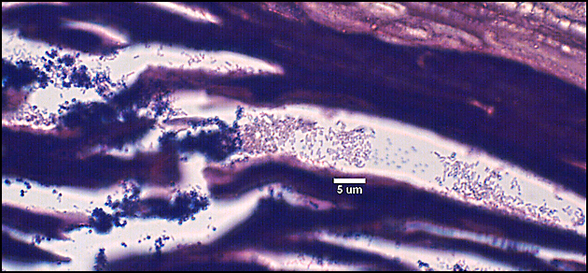
Biofilms composed of Gram-negative rods (Figure 8) and mixed-species biofilms (Figure 9) were also observed. These results were confirmed by results of culture and molecular analysis, discussed below, indicating that wound biofilms are indeed polymicrobial.
Overall, 30 out of 50 chronic wounds and one in 16 acute wounds were characterized as containing biofilm. Using Fisher’s exact test, this is a statistically significant difference (P>0.001) and indicates biofilms appear to be prevalent in chronic and rare in acute wounds.
Characterization of microorganisms present in wounds. From the patients involved in the biofilm study, described above, culture data was available for 37 of the chronic wounds and 5 of the acute wounds. Based on these data, bacteria from eight genera were frequently (>10%) isolated from wound samples (Table 1).
| Genus | Chronic % | Acute % |
| Staphylococcus | 65 |
60 |
| Enterococcus | 62 |
80 |
| Pseudomonas | 35 |
20 |
| Proteus | 24 |
20 |
| Citrobacter | 24 |
20 |
| Enterobacter | 24 |
20 |
| Streptococcus | 22 |
0 |
| Escherichia | 14 |
0 |
| Morganella | 8 |
0 |
| Klebsiella | 5 |
0 |
| Acinetobacter | 5 |
0 |
| Serratia | 3 |
0 |
| Xanthomonas | 3 |
0 |
In agreement with microscopic analysis, coccoid cells (Staphylococcus, Enterococcus) predominated in the wounds, although bacilli were also present. Strictly anaerobic bacteria implicated in previous studies of chronic wounds (Bowler and Davies, 1999) not cultured from chronic wounds despite specific attempts by two clinical laboratories to culture these organisms. However, the presence of facultative anaerobes, such as coliforms and fecal streptococci, in chronic wounds and not in acute wounds corroborates the findings of the previous studies. Culture-based methods for the identification of microorganisms have been the mainstay of clinical microbiology and will likely remain an important tool for the diagnosis and treatment of disease. For example, the isolation of specific strains from patients can provide important antibiotic sensitivity information to help guide treatment regimens. Nonetheless, the presence of viable but non-culturable bacteria and inability to culture many bacterial species under standard laboratory conditions necessitates the use of non-culture-based methods for the accurate diagnosis of disease.
